Nitrate Pollution Removal and Sustainable Energy Recovery
Nitrate is one of the most widespread water pollutants globally, posing a serious threat to drinking water safety and human health. The electrochemical conversion of nitrate into ammonia not only reduces nitrate pollution but also produces valuable ammonia. This provides a sustainable pathway to restore the balance of the global nitrogen cycle and offers technical support for the environmental and economic impacts of sustainable ammonia synthesis. However, developing electrode materials with low cost, high activity, and selectivity remains a key challenge in this field.
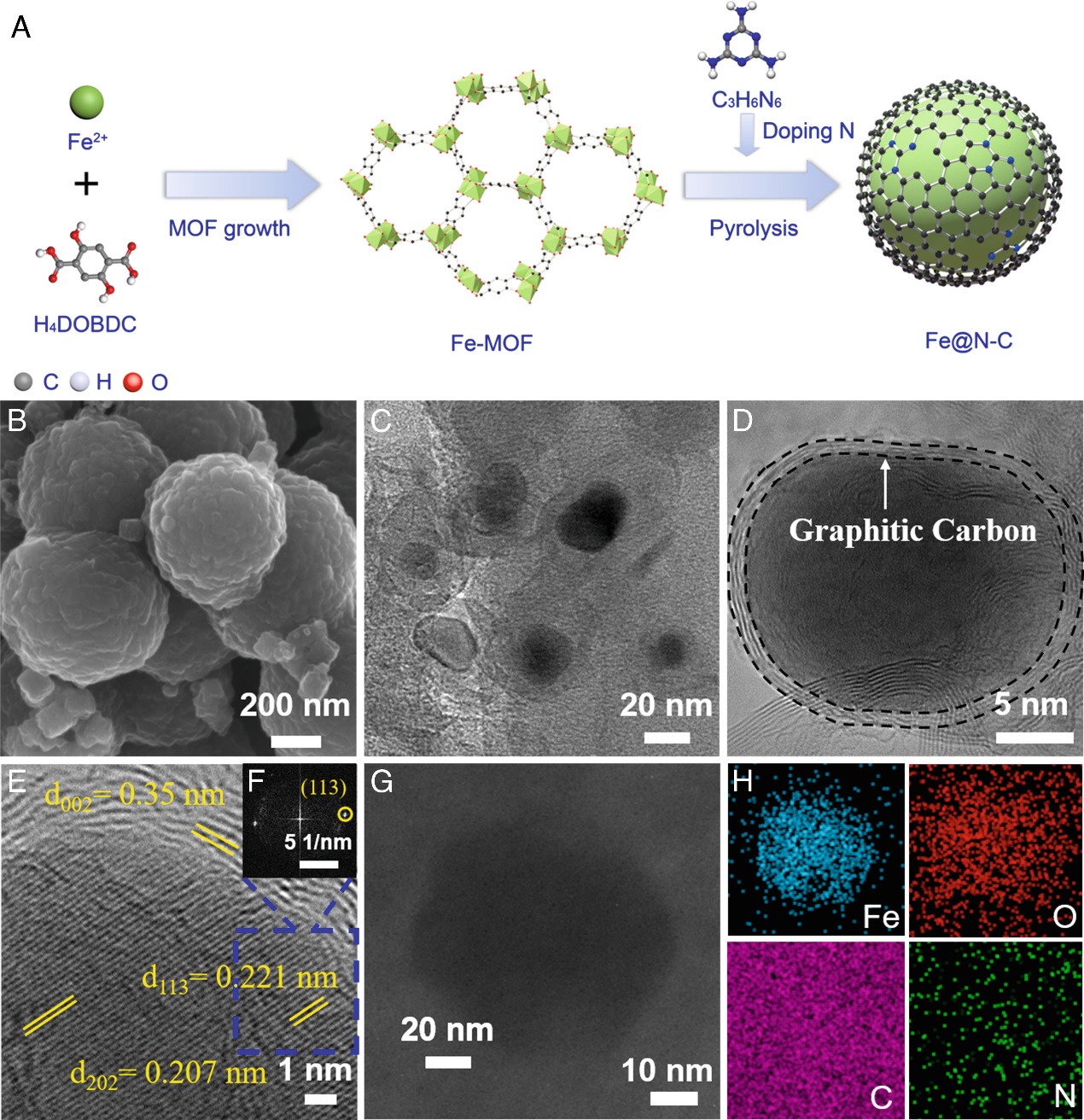
Associate Professor Miao Li's research group at the School of Environment, Tsinghua University addressed the bottleneck issue of weak adsorption capacity between carbon active sites and reactant molecules, which undermines reaction kinetics, reduces removal efficiency and selectivity, and hinders practical application. By innovating the theory and method of microstructure regulation for heterogeneous interfaces, the team constructed a catalyst with new active sites through nitrogen doping. This activated adjacent carbon atoms and enhanced electron transfer from metal to carbon, resulting in high catalytic activity. The team developed a metal-organic framework (MOF)-derived nitrogen-doped carbon-iron heterostructure (Fe@N~10~-C) electrocatalyst for electrochemical nitrate removal and ammonia energy production. The study achieved a nitrate removal efficiency of 125.8±0.5 mg N g~cat~^−1^ h^−1^ and an ammonia selectivity of nearly 100% (99.7±0.1%), the highest reported in existing research.
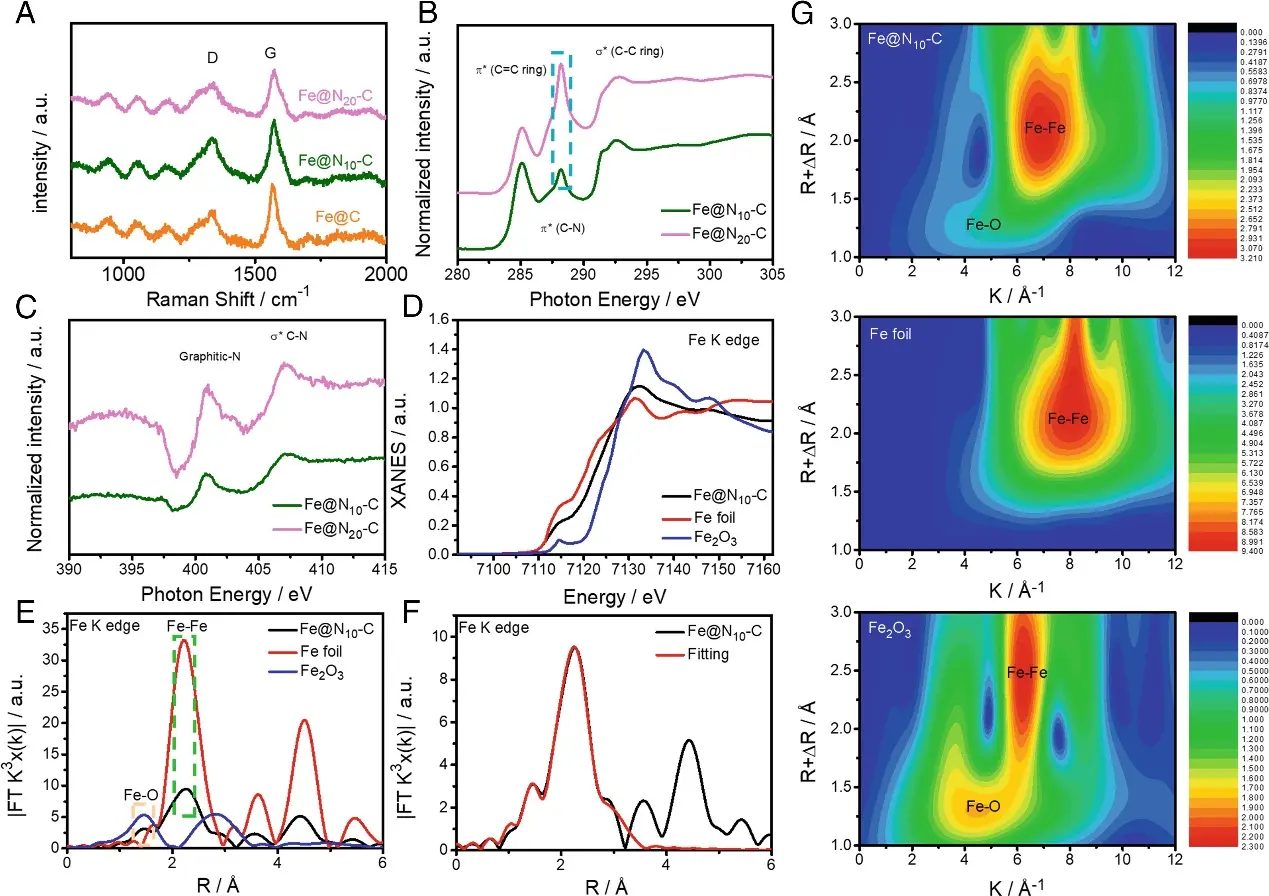
The study used synchrotron radiation to analyze the valence states and coordination environments of Fe, N, and C sites in the Fe@N~x~-C catalyst, further confirming the formation of a nitrogen-doped carbon structure during pyrolysis. After pyrolysis, the primary nitrogen species in the Fe@N~x~-C catalyst was graphitic nitrogen. The main active sites in Fe@N~x~-C were the C active sites adjacent to N sites (C~N~). The enhanced activity of the Fe@N~10~-C catalyst was attributed to the appropriate amount of nitrogen dopants in the carbon layers surrounding the Fe nanoparticles (NPs).
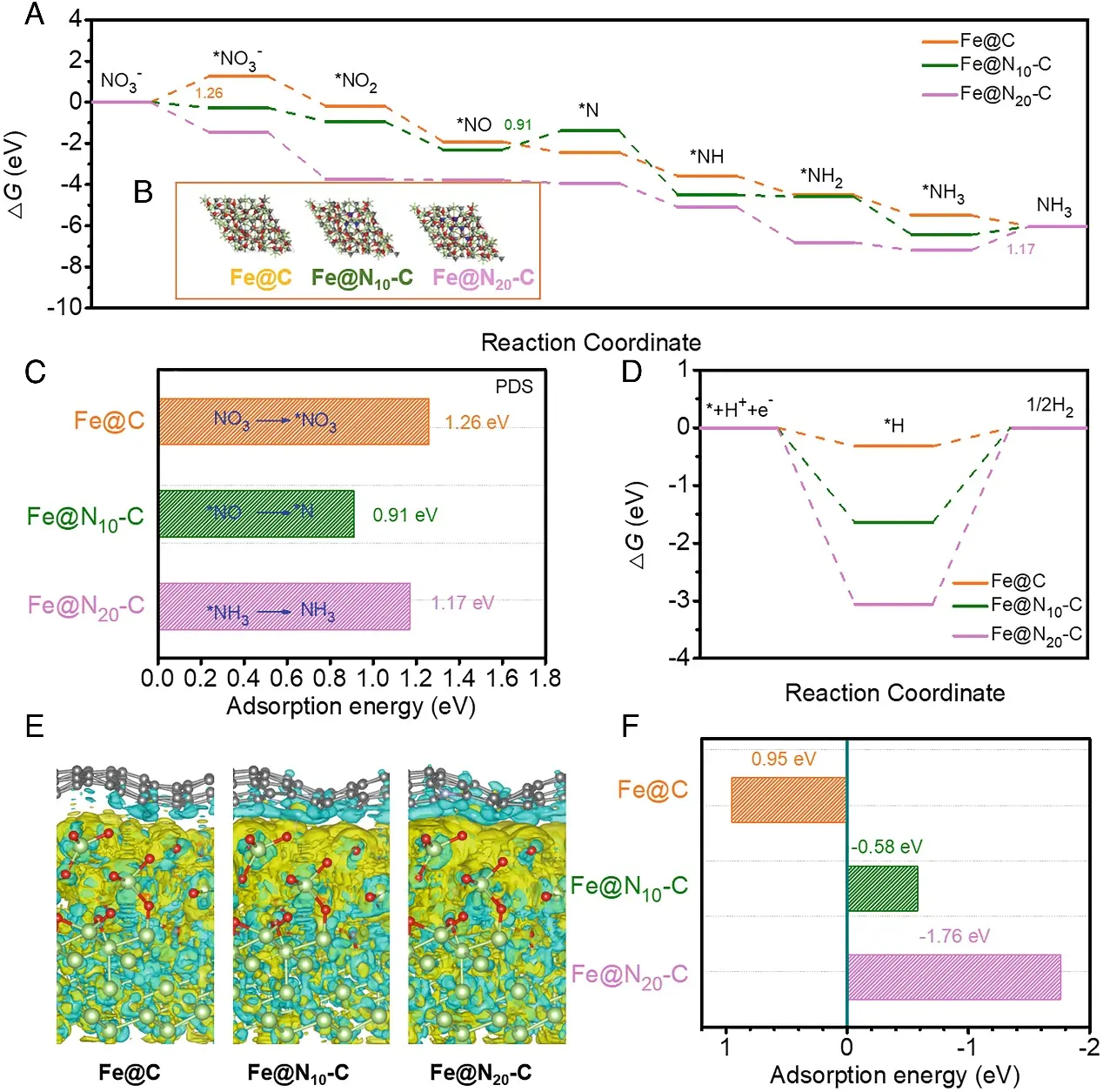
To further investigate the role of nitrogen in activating C atoms and improving the electrochemical nitrate reduction activity of Fe@N~10~-C, the study conducted density functional theory (DFT) calculations. Differential charge diagrams revealed the active sites of three representative models—Fe@N, Fe@N~10~-C, and Fe@N~20~-C—confirming that charge transfer occurred at the C~N~ sites and that N doping caused distortion in adjacent C atoms. The researchers then examined the effect of N doping on electronic structure. Greater charge accumulation was observed at the C~N~ sites, indicating that N doping increased charge density, thereby promoting NO~3~^−^ adsorption. Bader charge analysis also demonstrated greater charge transfer at the C~N~ sites, further confirming them as the active sites. To better understand the activation of C atoms and the origin of the Fe@N~10~-C catalyst's activity, the team studied the interaction between Fe and N-doped carbon. Differential charge diagrams showed that N doping influenced electron transfer from Fe NPs to N-doped carbon, activating C atoms, which in turn enhanced the adsorption of reactant molecules and improved reaction kinetics.